|
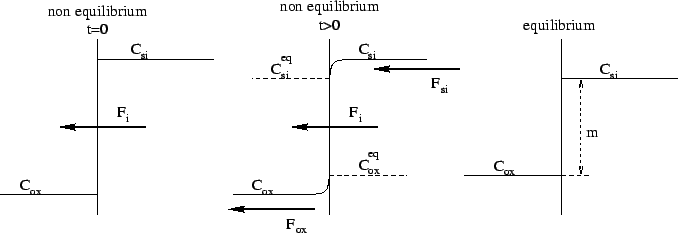
At a material interface a jump of the dopant concentration can often be established, even in thermodynamic equilibrium. This phenomenon is called segregation effect and can be observed with all species which are used in silicon techniques (boron, arsenic, etc.).
In general the dopant concentration within the two materials is not in the equilibrium thus a resulting flux over the interface tries to change the concentration towards equilibrium (Fig. 2.1).

|
In case of equilibrium it is usual to write
In case of non equilibrium a diffusion flux over the interface results
trying to balance the dislocation of the dopants. The equation
for the flux can be written as
| Fs = h . ( |
(2.22) |