Next: Second Law of Thermodynamics Up: 2.2 Heating Phenomena Previous: Zeroth Law of Thermodynamics Contents
The first law of thermodynamics has been first proposed by MAYER2.8 in
1841 [74]: ``Heat is a kind of energy and can therefore neither be
created nor destroyed.'' Hence, heat has been defined as the transfered thermal
energy between two systems if they are brought into thermal
contact [75]. The infinitesimal change of the internal energy ![]() of a system
of a system
![]() can be expressed as
can be expressed as
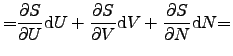 |
|||
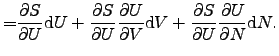 |
(2.29) |
| (2.33) | |||
| (2.34) |