Bismuth telluride
![]() , a narrow bandgap semiconductor, is one of the most commonly used thermoelectric materials [5]. It has been shown that by adding antimony telluride
, a narrow bandgap semiconductor, is one of the most commonly used thermoelectric materials [5]. It has been shown that by adding antimony telluride
![]() and bismuth selenide
and bismuth selenide
![]() to
to
![]() , it is possible to obtain a
, it is possible to obtain a ![]() of around unity. In turn, it was shown that by alloying of
of around unity. In turn, it was shown that by alloying of
![]() with bismuth selenide and antimony telluride, the thermal conductivity decreases, without significant degradation of the electrical conductivity [3]. As mentioned in
Sec. 1.2, working at high temperature is advantageous for energy conversion efficiency. However, the alloys of bismuth telluride are not suitable at temperature higher than
with bismuth selenide and antimony telluride, the thermal conductivity decreases, without significant degradation of the electrical conductivity [3]. As mentioned in
Sec. 1.2, working at high temperature is advantageous for energy conversion efficiency. However, the alloys of bismuth telluride are not suitable at temperature higher than
![]() . Common thermoelectric materials for higher temperatures are lead telluride
. Common thermoelectric materials for higher temperatures are lead telluride
![]() and silicon-germanium alloys. These materials have
and silicon-germanium alloys. These materials have ![]() between 0.5 and 1.1, depending on the temperature and the type of material (whether
between 0.5 and 1.1, depending on the temperature and the type of material (whether ![]() -type or
-type or ![]() -type) [3]. As a result, the average efficiency of current thermoelectric generators is about
-type) [3]. As a result, the average efficiency of current thermoelectric generators is about ![]() [6].
[6].
Good thermoelectric materials should simultaneously have a high Seebeck coefficient,
a high electrical conductivity, and a low thermal conductivity. While each property
of ![]() can individually be changed by several orders of magnitude, the interdependence
and coupling between these properties in bulk materials have made it extremely
difficult to increase
can individually be changed by several orders of magnitude, the interdependence
and coupling between these properties in bulk materials have made it extremely
difficult to increase ![]() . In the case of bulk materials, assuming the effective mass approximation and Fermi-Dirac statistics, one can relate the Seebeck coefficient and the electrical conductivity
. In the case of bulk materials, assuming the effective mass approximation and Fermi-Dirac statistics, one can relate the Seebeck coefficient and the electrical conductivity ![]() to the
carrier concentration
to the
carrier concentration ![]() as [6]:
as [6]:
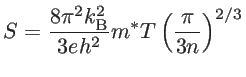 |
(1.14) |
| (1.15) |
| (1.16) |
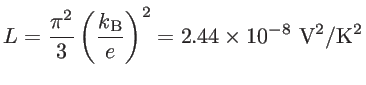 |
(1.17) |
The electrical conductivity is proportional to the carrier concentration, whereas the Seebeck coefficient is inversely proportional to the carrier concentration. Therefore, if one tries to increase ![]() by increasing
by increasing ![]() and thus
and thus ![]() , one may lose the gain through the reduction of
, one may lose the gain through the reduction of ![]() (and increase in
(and increase in ![]() ). Figure 1.6 schematically shows that insulators have high Seebeck coefficient and extremely low electrical conductivity, whereas metals have high electrical conductivity and a low Seebeck coefficient [7]. Therefore, in semiconducting materials a finite maximum of the thermoelectric power factor is achieved. As a result, the most effective way to enhance the thermoelectric figure of merit of bulk materials is to decrease the lattice contribution to the thermal conductivity
). Figure 1.6 schematically shows that insulators have high Seebeck coefficient and extremely low electrical conductivity, whereas metals have high electrical conductivity and a low Seebeck coefficient [7]. Therefore, in semiconducting materials a finite maximum of the thermoelectric power factor is achieved. As a result, the most effective way to enhance the thermoelectric figure of merit of bulk materials is to decrease the lattice contribution to the thermal conductivity ![]() . However, thermal conductivity reduction, without decreasing the power factor, was not possible for a long time (in all efforts up to the 1990s), and the
. However, thermal conductivity reduction, without decreasing the power factor, was not possible for a long time (in all efforts up to the 1990s), and the ![]() values were limited to unity. This translates to low conversion efficiencies and limited applications for thermoelectricity.
values were limited to unity. This translates to low conversion efficiencies and limited applications for thermoelectricity.