


Prev: 7.1.6 Process Control
Up: 7. High-Pressure Chemical Vapor
Next: 7.3 Application Examples
7.2 HPCVD of Tungsten
The model for the deposition of tungsten was derived from the
reduction of
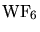 with hydrogen
with hydrogen
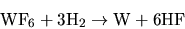 |
(7.6) |
forming 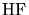 as by-product. The reaction results in three diffusion equations
for the gaseous species
as by-product. The reaction results in three diffusion equations
for the gaseous species
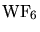 ,
, 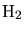 , and
, and 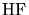 . Tungsten as reaction product is
directly deposited as solid at the wafer surface and therefore has not to be
considered for the diffusion. It is clear that the reaction chemistry is much
more complicated with different adsorbed or chemisorbed intermediates.
Furthermore the reduction can be carried out with a combination of hydrogen and
silane (
. Tungsten as reaction product is
directly deposited as solid at the wafer surface and therefore has not to be
considered for the diffusion. It is clear that the reaction chemistry is much
more complicated with different adsorbed or chemisorbed intermediates.
Furthermore the reduction can be carried out with a combination of hydrogen and
silane ( ). For demonstration purposes the chemistry will be restricted
to an overall formulation, even if more complex models can be formulated within
the Analytical Model Interface.
). For demonstration purposes the chemistry will be restricted
to an overall formulation, even if more complex models can be formulated within
the Analytical Model Interface.
The derivation of effective species diffusivities in mixtures, which depend on
composition, pressure and temperature uses the Chapman-Enskog equation and
the characteristic Lennard-Jones length 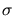 and energy
and energy
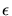 [30][35].
[30][35].
The deposition rate was experimentally found [43] to follow the
expression
where 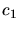 and
and 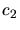 are experimentally determined constants,
are experimentally determined constants, 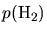 and
and
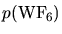 are hydrogen and
are hydrogen and
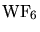 partial pressures and
partial pressures and 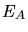 is the
activation energy which was set to 68.4 kJ/mol [77].
is the
activation energy which was set to 68.4 kJ/mol [77].
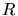 is the gas constant and
is the gas constant and 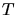 is the temperature.
This rate expression is substituted into (7.2) and couples the
concentrations of the three gaseous species by the stoichiometry of (7.6).
When tungsten is formed at the wafer surface a certain amount of
is the temperature.
This rate expression is substituted into (7.2) and couples the
concentrations of the three gaseous species by the stoichiometry of (7.6).
When tungsten is formed at the wafer surface a certain amount of
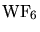 is consumed from the gas phase, thus reducing the concentration of
is consumed from the gas phase, thus reducing the concentration of
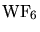 . The same applies to the hydrogen concentration, differing only in the
stoichiometric factor. Simultaneously
. The same applies to the hydrogen concentration, differing only in the
stoichiometric factor. Simultaneously 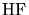 is formed and has to be added to
the
is formed and has to be added to
the 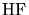 concentration in the gas phase.
concentration in the gas phase.



Prev: 7.1.6 Process Control
Up: 7. High-Pressure Chemical Vapor
Next: 7.3 Application Examples
W. Pyka: Feature Scale Modeling for Etching and
Deposition Processes in Semiconductor Manufacturing
![]() and energy
and energy
![]() [30][35].
[30][35].
![]()
![]()
![]()
![]()