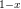4.1 Properties of Interconnect Materials
The full functionality of an IC cannot be reached with the use of a single isolated device. It
requires an engineered interconnected network, which enables the proper communication
between the different components (e.g. resistors, capacitors, diodes, transistors, and more).
The interconnections should behave akin to an ideal wire and minimize the disruption of the
signal flux inside the circuit.
Interconnections may be composed by conductive lines, vias, wires, pads, and joints [65].
Each of these components can be formed by different materials. Metals and theirs alloys are
usually the best choice for building interconnections, due to their high electrical
conductivity. In the very beginning of the semiconductor industry aluminum and copper
were the most common metals, but with the increasing demand for performance,
integration, and reliability a wider variety started to be employed, such as tin, tungsten,
zinc, gold, and nickel [66].
The introduction of metal structures inside semiconductor devices creates several problems.
For instance, metals have a large CTE when compared to silicon, therefore during
thermal variation, stresses arise due to the thermal mismatch between the materials.
Furthermore, the manufacturing process is also very challenging. Metals are usually
deposited by employing various methods, such as Chemical Vapor Deposition
(CVD), Physical Vapor Deposition (PVD), and their variations [38][66]. Each
method has its particularities regarding material quality, adhesion, and mechanical
properties, which also must be considered when attempting to generate reliable
devices[38].
To overcome all the challenges of an interconnection fabrication, the implemented materials
and their interactions with the silicon must be understood. Each part of an interconnection
demands a specific approach. In this work the focus stays with vias and the materials most
frequently found in a via structure.
Vias are the elements responsible for the vertical connections in a device. They make the
bridge between the different metal lines and, in the case of TSVs, between dies. Aluminium,
copper, and tungsten are the most common conduction metals, but there is a large
assortment of other materials required to support via fabrication. Some of these materials
and their purpose are present in Table 4.1.
| Table 4.1.: | Melting point and electrical resistivity of common interconnection
materials. The materials are grouped by their function within an interconnect
structure. |
The function of each type of material can be summarized in the following:
- Silicides are used as a conductive material only in local regions of the structure,
which have to be exposed to high temperatures or oxidizing environment.
- Barriers are applied to prevent diffusion of metal atoms toward silicon,
minimizing the risk of contamination and current leakage.
- Glues are needed to improve the adhesion of metal films in the silicon surface,
avoiding delamination problems which could lead to a via failure.
- Passivation materials are protective coating against the hazards of the ambient.
As seen in Table 4.1, copper is the most suitable material regarding electrical conductivity,
but it was not used in large scale production as the conductive material in a via until 1997.
Several challenges were present which limited copper deposition, such as patterning
difficulties and silicon contamination. Volatile copper compounds were unknown and the
technology at the time required such compounds for metal deposition [74]. The problem was
overcome through the invention of the dual damascene process which patterns
the silicon itself by etching and filling the created trenches and vias with copper
[74][75][76].
Silicon contamination was solved by the application of barriers metals (e.g. Ta, TaN), which
must prevent copper diffusion without drastically reducing the conductivity, otherwise there
is no benefit for copper utilization[75][76][77].
Before 1997, tungsten (vias) and aluminium (lines) were the dominant interconnection
materials [66]. The processing of Al-W interconnections is easier in comparison to the
processes required to generate copper based vias [66]. They do not face the severe problems
of silicon contamination and volatile compounds are known and available. Moreover,
aluminium and tungsten mechanical properties are more compatible with silicon, i.e.,
aluminium and tungsten enjoy a lower CTE mismatch and higher melting point than
copper.
The effort required to incorporate high conductivity metals is justified only for some
industrial applications. The high conductivity metal allows for the creation of smaller
and more energy efficient devices, a very important feature in general purpose
processors.
The idea behind a TSV is not different from the traditional via. Both are vertical
connections, but the dimension of the TSVs are much larger in comparison to the metal line
vias. Therefore, mechanical properties become much more relevant than before. The
manufacturing of TSVs demand a more careful design to manage the increasing mechanical
instability of the structure.
 C)
C)  /cm)
/cm) 


 W
W