Dynamic Clustering (Model DIFDCL)




Next: Static Clustering (Model
Up: 3.2.2 High Concentration Effects
Previous: 3.2.2 High Concentration Effects
With immobile impurity clusters composed of 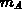 impurity atoms (
impurity atoms (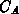 ) and
) and
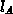 electrons (
electrons ( ), the kinetic equation for the clustering process is
given by (3.2-11). The coefficients
), the kinetic equation for the clustering process is
given by (3.2-11). The coefficients 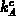 and
and 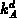 are the
clustering and declustering rates, respectively. Note, that one impurity
cluster
are the
clustering and declustering rates, respectively. Note, that one impurity
cluster 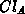 contains
contains 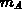 clustered impurity atoms (
clustered impurity atoms (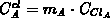 ).
).
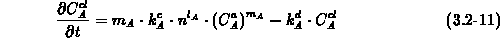
The model DIFDCL takes into consideration the dynamic clustering
of arsenic [Tsa80]. For arsenic predominant clusters consist of three
arsenic atoms and one electron [Gue82] which are active at the
diffusion temperature and electrically neutral at room temperature
[Tsa80], giving 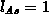 and
and 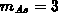 . All dopants
except arsenic are assumed active and mobile (3.2-12). Although
attempts for kinetic modeling of boron cluster formation exist
(e.g. [Cow90], [Sol90]), the models and parameters are not
well-established.
. All dopants
except arsenic are assumed active and mobile (3.2-12). Although
attempts for kinetic modeling of boron cluster formation exist
(e.g. [Cow90], [Sol90]), the models and parameters are not
well-established.
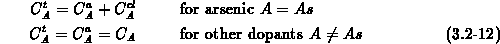
We solve equation (3.2-13) for all impurities other than
arsenic, and equations (3.2-14) and (3.2-15)
for mobile arsenic and clustered arsenic, respectively.
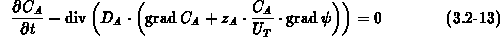
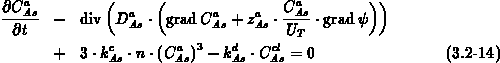
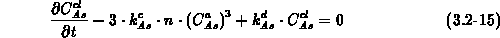
According to the charge state of the impurities (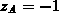 for singly
charged acceptors,
for singly
charged acceptors, 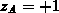 for singly charged donors) and for an
arsenic atom in a cluster (
for singly charged donors) and for an
arsenic atom in a cluster (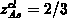 ) the net active
concentration
) the net active
concentration 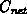 (at diffusion temperature), the electron
concentration
(at diffusion temperature), the electron
concentration  and the electric field are given by (3.2-16),
(3.2-17) and (3.2-18), respectively.
and the electric field are given by (3.2-16),
(3.2-17) and (3.2-18), respectively.
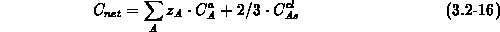
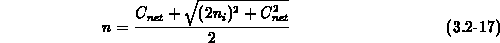
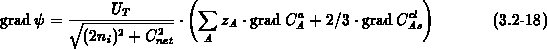
Neumann boundary conditions (zero flux 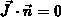 ) are
used for all impurities at all boundaries.
) are
used for all impurities at all boundaries.
Martin Stiftinger
Wed Oct 19 13:03:34 MET 1994
 impurity atoms (
impurity atoms (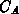 ) and
) and
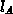 electrons (
electrons ( ), the kinetic equation for the clustering process is
given by (3.2-11). The coefficients
), the kinetic equation for the clustering process is
given by (3.2-11). The coefficients 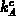 and
and 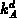 are the
clustering and declustering rates, respectively. Note, that one impurity
cluster
are the
clustering and declustering rates, respectively. Note, that one impurity
cluster 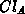 contains
contains 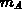 clustered impurity atoms (
clustered impurity atoms (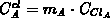 ).
).




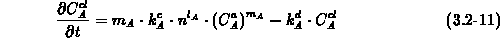
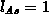 and
and 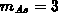 . All dopants
except arsenic are assumed active and mobile (
. All dopants
except arsenic are assumed active and mobile (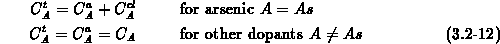
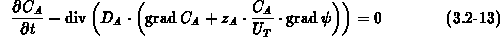
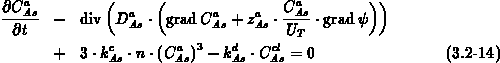
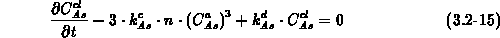
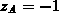 for singly
charged acceptors,
for singly
charged acceptors, 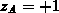 for singly charged donors) and for an
arsenic atom in a cluster (
for singly charged donors) and for an
arsenic atom in a cluster (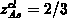 ) the net active
concentration
) the net active
concentration 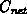 (at diffusion temperature), the electron
concentration
(at diffusion temperature), the electron
concentration 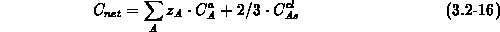
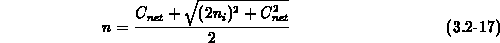
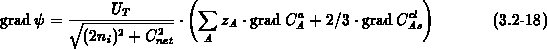
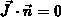 ) are
used for all impurities at all boundaries.
) are
used for all impurities at all boundaries.