


Next: 5.3 Double Layer
Up: 5.2 Charge Transport in
Previous: 5.2.1 Bulk Transport
5.2.2 Interface Transport
Despite the fact that the mobile charge carriers in the bulk of an electrolyte behave similar to the electrons in a resistor, the situation changes at surfaces. At least one metal-electrolyte interface is present in nearly all electrochemical experiments. The metal contact is usually called electrode. Compared to the charge transport phenomena in the bulk of the electrolyte or in the bulk of the electrode, the metal-electrolyte interface exhibits a quite deviating behavior. The charge carriers from the solute are not able to migrate into the metal and the electrons from the metal can not migrate into the solute without assistance. The only way to pass an electric current from the metal into the solute is a charge exchange at the interface via an electron transfer from the electrode to the ions in the liquid [190,192]. This exchange can be utilized with a redox-reaction at the surface of the metal electrode. There are three distinguishable cases for electrodes [192]:
- Inert Metal Electrodes. One can think of them as ideally polarizeable electrodes. These metals show high resistance to redox reactions and thus inhibit electronic transfer. This type of electrode-electrolyte interface does not allow charge transfer between the two phases and is equivalent to a capacitor.
- Non-Polarizable Electrodes. This type of electrodes do not exhibit any resistance to charge transfer from the electrode to the solute and therefore do not show charge accumulation at the interface. This characteristic is modeled with a short circuit or an extremely small resistor.
- Partially Polarizable Electrodes. These electrodes feature distinct levels of charge hindrance to the movement of the charge carriers and require a quite elaborate description of the interface.
Normally, metal-semiconductor electrodes are neither non-polarizable nor ideally polarized. Over a range of certain potentials, an interface can posses the properties of an ideally polarizable electrode, and outside of these potential intervalls behave completely different[193]. The concept of an equilibrium potential is the key to describe the electric transport through the electrode interface. Every time an electrode is brought into contact with an electrolyte, redox-reactions take place and result in a certain amount of net charge in the electrolyte interface region and on the electrodes interface. This is caused by the two different chemical potentials of the two phases [194]. The charge separation builds up an opposing electric field at the interface, thus blocking further redox-reactions. In conjunction with the excess charge a constant electrostatic potential will appear between the interface and the electrode, unless an external circuit or any other means of external charge removing process occurs. This potential can only be determined indirectly by measuring relative to another electrode. Normally, a standardized hydrogen electrode is employed as reference electrode and any standard electrode potential is measured against it[190].
Applying an external bias between the electrode and the electrolyte causes a fraction of the potential to oppose the built-in electrical potential and the rest of it, the so called overpotential, enables current transport through the interface. Tafel's equation [193,192] is a simplified model for the 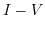 characteristics:
characteristics:
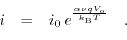 |
(5.10) |
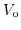 denotes the overpotential value,
denotes the overpotential value, 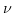 is the valence of the ionic species,
is the valence of the ionic species, 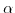 describes a constant depending on the electrode's transfer kinetics, and
describes a constant depending on the electrode's transfer kinetics, and 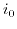 stands for a multiplicative constant related to the reaction rate and the exposed surface area. This description is only applicable for small voltages. Exposure to higher biases can open up paths for different phenomena and differing
stands for a multiplicative constant related to the reaction rate and the exposed surface area. This description is only applicable for small voltages. Exposure to higher biases can open up paths for different phenomena and differing 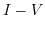 profiles can be observed[193].
profiles can be observed[193].



Next: 5.3 Double Layer
Up: 5.2 Charge Transport in
Previous: 5.2.1 Bulk Transport
T. Windbacher: Engineering Gate Stacks for Field-Effect Transistors
![]() characteristics:
characteristics: