


Next: 5.8.2 Sensing via Catalysts
Up: 5.8 The ISFET as
Previous: 5.8 The ISFET as
ISFETs facilitate the detection of ions in a relatively easy way, thus, this application is most commonly used. The device relies on the chemical interaction between the surface binding sites and the hydrogen ions as explained in Section 5.6. This pH sensitivity was also the initial application by Bergveld [203]. The change in the flatband voltage per pH change in the solution determines the sensitivity of the ISFET device. The major criteria of the sensitivity of the oxide surfaces are determined by the surface binding site density 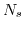 and the forward and backward reaction constants
and the forward and backward reaction constants 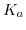 and
and 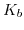 (5.21). Table 5.2 shows some sensitivity values for different commonly used gate dielectric materials.
Therefore, one can produce an efficient pH sensor by utilizing the open surface binding sites for detecting the dissolved protons. However, if large dissolved ions are desired for detection, due to their water shell, they cannot approach the interface closer than the OHP and thus the surface binding approach is not feasible. This problem can be circumvented by preparing a reaction medium in the ISFET gate stack such that the target species will generate excess hydrogen ions via a chemical reaction and thus change the pH of the solution. This method is particularly appealing for acid-base reactions, where the pH of the solution is directly affected by the reaction rate.
(5.21). Table 5.2 shows some sensitivity values for different commonly used gate dielectric materials.
Therefore, one can produce an efficient pH sensor by utilizing the open surface binding sites for detecting the dissolved protons. However, if large dissolved ions are desired for detection, due to their water shell, they cannot approach the interface closer than the OHP and thus the surface binding approach is not feasible. This problem can be circumvented by preparing a reaction medium in the ISFET gate stack such that the target species will generate excess hydrogen ions via a chemical reaction and thus change the pH of the solution. This method is particularly appealing for acid-base reactions, where the pH of the solution is directly affected by the reaction rate. 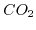 sensing in an electrolytic solution is a good example of this type of mechanism [209]. Carbon dioxide
sensing in an electrolytic solution is a good example of this type of mechanism [209]. Carbon dioxide 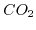 reacts with water producing carbon acid and hydroxyl ions. The process is governed by the following reaction:
reacts with water producing carbon acid and hydroxyl ions. The process is governed by the following reaction:
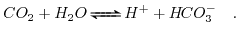 |
(5.30) |
Utilizing this type of reactions enables the ISFET to detect different kinds of ions via the hydrogen dependent reaction at the empty dangling bonds of the oxide surface. With this type of sensor the ions are not sensed directly, because they can not interact with the surface. Only the excess hydrogen ions caused through the chemical reaction at the solvation of the desired ions will react with the dielectric interface.
Many different ionic sensors are based on their tendency to change the pH of a solution (act as a base or acid in an aqueous solution). In order to maintain the specifity of the sensor one has to take care that other eventually present ions do not contribute. Any change in the pH of the solution must be provoked from reactions concerning the target ions. This can be accomplished by adding a number of membranes to the ISFET chip, which are only transparent to the desired target ion [199] (as illustrated in Fig. 5.6). Applying this kind of additional layer, only the target ions will be able to reach the gate insulator and contribute to the local pH of the solution at the gate insulator interface.
Figure 5.6:
Introducing a membrane into the ISFET hinders unwanted ion-species to diffuse to the insulator interface. Thus, only the selected ion species can approach the gate insulator, and the selectivity of the ISFET is ensured.
|
|
Table 5.2:
Sensitivity of ISFET devices for different gate dielectrics.
| Sensitive layer |
pH range |
Sensitivity
![$ [\frac{mV}{pH}]$](img799.png) |
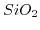 |
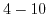 |
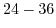 [210] [210] |
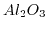 |
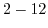 |
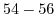 [211] [211] |
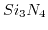 |
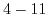 |
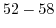 [1] [1] |
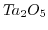 |
 |
 [211] [211] |



Next: 5.8.2 Sensing via Catalysts
Up: 5.8 The ISFET as
Previous: 5.8 The ISFET as
T. Windbacher: Engineering Gate Stacks for Field-Effect Transistors
![\includegraphics[width=0.4\textwidth]{figures/isfet_membrane.ps}](img798.png)