| |
Si |
4H-SiC |
6H-SiC |
3C-SiC |
|---|
Bandgap energy
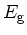 |
|---|
| [eV] |
|
1.12 |
3.26 |
3.03 |
2.4 |
| Relative dielectric |
|---|
constant
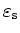 |
|---|
|
11.9 |
9.7 |
9.66 |
9.72 |
Breakdown Field
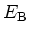 |
|---|
@ N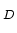 =10 =10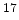 cm cm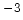 [MV/cm] [MV/cm] |
|
0.3 |
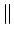 c-axis: 3.0 c-axis: 3.0 |
|
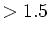 |
| Thermal Conductivity |
|---|
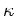 [W/cmK] [W/cmK] |
|
1.31 |
4.9 |
4.9 |
3.2 |
| Intrinsic Carrier |
|---|
Concentration
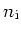 [cm [cm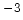 ] ] |
|---|
|
9.65
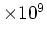 |
5
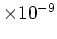 |
1.6
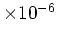 |
1.5
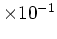 |
Electron Mobility
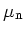 |
|---|
@ N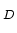 =10 =10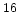 cm cm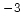 [cm [cm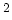 /Vs] /Vs] |
|
1430 |
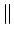 c-axis: 900 c-axis: 900 |
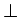 c-axis: 800 c-axis: 800 |
|
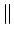 c-axis: 60 c-axis: 60 |
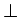 c-axis: 400 c-axis: 400 |
|
800 |
Hole Mobility
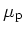 |
|---|
@ N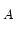 =10 =10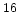 cm cm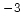 [cm [cm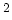 /Vs] /Vs] |
|
480 |
115 |
90 |
40 |
| Saturated Electron |
|---|
Velocity 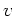 [10 [10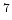 cm/s] cm/s] |
|---|
|
1 |
2 |
2 |
2.5 |
| Donors & Ionization |
|---|
Energy
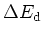 [meV] [meV] |
|---|
|
|
|
|
N: 50 |
| Acceptors & Ionization |
|---|
Energy
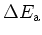 [meV] [meV] |
|---|
|
|
|
|
Al: 270 |
| 2003 Commercial |
|---|
| Wafer Diameter [inches] |
|---|
|
12 |
3 |
3 |
 |