Next: 2.2 Heating Phenomena Up: 2. Thermal Effects in Previous: 2. Thermal Effects in Contents
To fully describe a complex system on a microstate level, an enormous number of
different microstates has to be known, and their interactions have to be
determined in order to obtain the future behavior from the past states.
Because is not possible to store the bulk of data that would be necessary to
calculate all the effects correctly, a statistics-based description has to be
used [70].
A possible way to obtain a representative quantity is to count the number of
occupied or unoccupied microstates.
Historically, the maximum number of possible states which can be
theoretical occupied was chosen to determine the disorder of a system.
This maximum value of disorder correlates with the energy of the system.
Because the number of microstates is always a positive integer and is normally
enormously large, the corresponding information content of a given system
![]() can be logarithmically counted according to information theory.
This introduced logarithmic quantity is the so-called entropy
can be logarithmically counted according to information theory.
This introduced logarithmic quantity is the so-called entropy
![]() which represents the level of maximum disorder.
which represents the level of maximum disorder.
Hence, the historical definition of the entropy of a given system is a measure
for the number of all possible quantum states which can be achieved following a
uniform probability distribution. If the number of reachable microstates for a
system
![]() is determined by
is determined by
![]() , the
corresponding entropy
, the
corresponding entropy ![]() of this system is defined as its natural
logarithm
of this system is defined as its natural
logarithm
| (2.19) |
If two systems are considered as spatially and thermally insulated systems
![]() and
and
![]() , where each of them has a certain
internal energy
, where each of them has a certain
internal energy ![]() and
and ![]() as shown
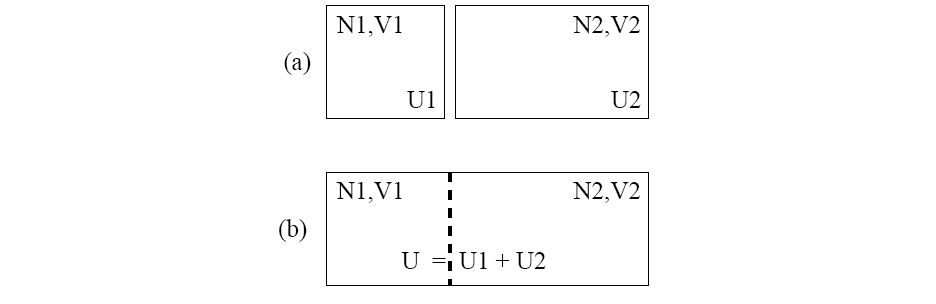
in Figure 2.1a. and if they are brought into thermal contact
(cf. Figure 2.1b), the number of particles and the volumes remain
constant, but the individual energies
as shown
in Figure 2.1a. and if they are brought into thermal contact
(cf. Figure 2.1b), the number of particles and the volumes remain
constant, but the individual energies ![]() and
and ![]() are no
longer spatially confined [69].
Therefore, an energy transmission can be observed. In this case, the total energy
are no
longer spatially confined [69].
Therefore, an energy transmission can be observed. In this case, the total energy
![]() remains constant if no other energy fluxes
are observed.
So the energy flows in the most probable case from one side to the other under
the constraint that the product of the single entropies
remains constant if no other energy fluxes
are observed.
So the energy flows in the most probable case from one side to the other under
the constraint that the product of the single entropies
![]() maximizes.
That is again a measure for the total number of states of the global system and
therefore, also the sum
maximizes.
That is again a measure for the total number of states of the global system and
therefore, also the sum
| (2.20) |
 |
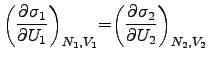 |
(2.21) |
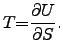 |
(2.25) |
An interesting corollary to definition (2.22) is the fact that the value zero for the fundamental temperature cannot be reached under the constraint of finite energy resources because the energy gradient would become infinity which has been proven to be impossible [71].