

Next:3.
Numerical Discretization MethodsUp:2.2
OxidationPrevious:2.2.2
Point Defect Models
2.2.3 Segregation Effects Arising
at the Moving Interface
In contrast to the segregation effect described in the previous
section where the interface is assumed to be stationary, now an additional
effect namely the moving interface due to oxidation phenomena has to be
taken into account (Fig. 2.4):
Figure 2.4: Model of a segregation effect at the moving
interface between silicon and silicon dioxide
 |
Ignoring the mass transport phenomena described in Section
2.1.3,
which is equivalent to the conditions Fi = 0, Fsi
= 0, and Fox = 0, the
concentration Coxnew
would be proportional to the interface concentration Csieq
Coxnew = 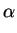 . Csieq
. Csieq |
|
|
(2.44) |
where 
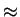 0.44. This corresponds with the fact that under equilibrium conditions
also
0.44. This corresponds with the fact that under equilibrium conditions
also
| Csieq = m
. Coxnew |
|
|
(2.45) |
must be fulfilled causing m
= 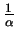 .
Under normal circumstances this is not true. Depending on the species different
ratios between m and
.
Under normal circumstances this is not true. Depending on the species different
ratios between m and  have been found (Fig. 2.5):
have been found (Fig. 2.5):
-
Boron (m <
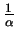 ):
to the flux Fi via the interface the flux Fc
due to silicon consumption is superpositioned and intensified. Thus boron
tends to move towards the oxide layer and declines in the silicon layer.
):
to the flux Fi via the interface the flux Fc
due to silicon consumption is superpositioned and intensified. Thus boron
tends to move towards the oxide layer and declines in the silicon layer.
-
Arsenic and phosphorus (m
> >
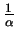 ):
in contrast to boron the dopant concentration of arsenic and phosphorus
is increasing at the silicon interface and a typical pile-up can
be observed.
):
in contrast to boron the dopant concentration of arsenic and phosphorus
is increasing at the silicon interface and a typical pile-up can
be observed.
Figure 2.5: Segregation effects of arsenic, boron and phosphorus
at the moving interface between silicon and silicon dioxide
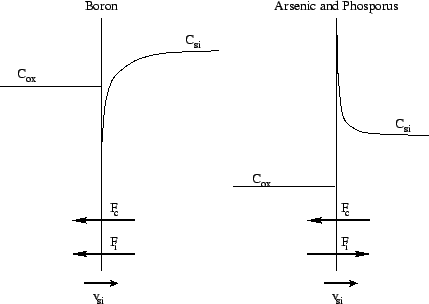 |
The following general rules can be estimated for the segregation
effect during an oxidizing process:
-
the segregation effect increases in case of lower temperatures since the
oxidation velocity decreases slower than the diffusion coefficients do.
-
the higher the diffusion coefficient of a species in the silicon layer
the faster non equilibrium states can be reduced, but the farther reaches
the effect into the layer.
-
wet oxidation favors the segregation effect because of higher oxidation
velocity.


Next:3.
Numerical Discretization MethodsUp:2.2
OxidationPrevious:2.2.2
Point Defect Models
Mustafa Radi
1998-12-11


![]()
![]() 0.44. This corresponds with the fact that under equilibrium conditions
also
0.44. This corresponds with the fact that under equilibrium conditions
also
![]() .
Under normal circumstances this is not true. Depending on the species different
ratios between m and
.
Under normal circumstances this is not true. Depending on the species different
ratios between m and ![]() have been found (Fig. 2.5):
have been found (Fig. 2.5):
