Subsections
2.2.1 Kinetics and Growth of Silicon Dioxide
The main ambient parameter used to control oxide growth during silicon oxidation is temperature. However, it is also
possible to vary the hydrostatic pressure in the reaction chamber. Whether the oxidation environment is wet (H O) or
dry (O
O) or
dry (O ) also plays a role in determining the growth rate, in addition to the role played by the
crystal orientation of the silicon wafer.
) also plays a role in determining the growth rate, in addition to the role played by the
crystal orientation of the silicon wafer.
During dry oxidation, the wafer is placed in a pure oxygen gas (O ) environment and the chemical reaction which ensues
is between the solid silicon atoms (Si) on the surface of the wafer and the approaching oxide gas
) environment and the chemical reaction which ensues
is between the solid silicon atoms (Si) on the surface of the wafer and the approaching oxide gas
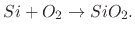 |
(29) |
Figure 2.7:
Oxide thickness versus oxidation time for dry (O ) oxidation of a (100) oriented silicon
wafer under various temperatures.
) oxidation of a (100) oriented silicon
wafer under various temperatures.
|
![\includegraphics[width=0.65\linewidth]{chapter_oxidation/figures/oxidation_100_dry.eps}](img158.png) |
Figure 2.7 shows the oxide thickness as a function of oxidation time for dry oxidation. It can be noted that the
oxidation rate does not exceed  150nm
150nm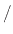 h, making it a relatively slow process which can be accurately controled in order
to achieve a desired thickness. The oxide films resulting from a dry oxidation process have a better quality than those
grown in a wet environment, which makes them more desirable when high quality oxides are needed. Dry oxidation is generally
used to grow films not thicker than 100nm or as a second step in the growth of thicker films, after wet oxidation has
already been used to obtain a desired thickness. The application of a second step is only meant to improve the quality of the
thick oxide.
h, making it a relatively slow process which can be accurately controled in order
to achieve a desired thickness. The oxide films resulting from a dry oxidation process have a better quality than those
grown in a wet environment, which makes them more desirable when high quality oxides are needed. Dry oxidation is generally
used to grow films not thicker than 100nm or as a second step in the growth of thicker films, after wet oxidation has
already been used to obtain a desired thickness. The application of a second step is only meant to improve the quality of the
thick oxide.
During wet oxidation, the silicon wafer is placed into an atmosphere of water vapor (H O) and the ensuing chemical reaction
is between the water vapor molecules and the solid silicon atoms (Si) on the surface of the wafer, with hydrogen gas (H
O) and the ensuing chemical reaction
is between the water vapor molecules and the solid silicon atoms (Si) on the surface of the wafer, with hydrogen gas (H )
released as a byproduct
)
released as a byproduct
 |
(30) |
Figure 2.8 shows the oxide thickness as a function of oxidation time for wet oxidation processing.
Figure 2.8:
Oxide thickness versus oxidation time for wet (H O) oxidation of a (100) oriented silicon
wafer under various temperatures.
O) oxidation of a (100) oriented silicon
wafer under various temperatures.
|
![\includegraphics[width=0.65\linewidth]{chapter_oxidation/figures/oxidation_100_wet.eps}](img160.png) |
It is evident that wet oxidation operates with much higher oxidation rates than dry oxidation, up to approximately 600nm/h.
The reason is the ability of hydroxide (OH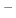 ) to diffuse through the already-grown oxide much quicker than
O
) to diffuse through the already-grown oxide much quicker than
O , effectively widening the oxidation rate bottleneck when growing thick oxides, which is the diffusion of species.
Due to the fast growth rate, wet oxidation is generally used where thick oxides are required, such as insulation and passivation
layers, masking layers, and for blanket field oxides.
, effectively widening the oxidation rate bottleneck when growing thick oxides, which is the diffusion of species.
Due to the fast growth rate, wet oxidation is generally used where thick oxides are required, such as insulation and passivation
layers, masking layers, and for blanket field oxides.
As the temperature in the oxidation environment is increased, the oxidation rate can increase significantly, both
in wet and dry processes. The temperature dependence on the oxidation rate can be observed in Figure 2.7 and
Figure 2.8 for dry and wet oxidation, respectively.
In Figure 2.9, the ratio between oxide thickness and temperature is visualized, suggesting the
existence of an exponential relationship between the thickness (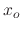 ) and inverse negative temperature
) and inverse negative temperature
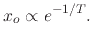 |
(31) |
The dramatic increase in oxide thickness with increasing temperature is not surprising, since the diffusivity (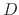 ) of
oxygen and water through the oxide depends greatly on temperature,
) of
oxygen and water through the oxide depends greatly on temperature,
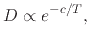 |
(32) |
where 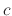 is a parameter independent of temperature
is a parameter independent of temperature 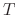 .
Since the oxidant diffusivity increases exponentially with increasing temperature, so should the oxidation rates, because the
diffusivity of oxidants is the rate-limiting step when thicker oxides (
.
Since the oxidant diffusivity increases exponentially with increasing temperature, so should the oxidation rates, because the
diffusivity of oxidants is the rate-limiting step when thicker oxides (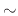 30nm) are grown. A higher diffusivity rate
means that more oxidants will be allowed to penetrate through the already grown oxide to reach the silicon surface.
30nm) are grown. A higher diffusivity rate
means that more oxidants will be allowed to penetrate through the already grown oxide to reach the silicon surface.
The effect of hydrostatic pressure on thermally grown oxides in dry and wet environments is shown in Figure 2.10a
and Figure 2.10b, respectively, while Figure 2.10c shows the direct relationship between the oxide
thickness and the applied pressure. It is evident that increasing the pressure results in thicker oxides and a faster
oxidation rate.
A logarithmic relationship appears to exist between the thickness of oxide grown and the applied pressure. The main advantage
of increasing the pressure during oxidation is to achieve relatively fast oxidation rates at reduced
temperatures [124], [179]. Reducing the processing temperature results in less impurities
and minimal movement of the junction during multiple subsequent oxidation steps required for complex IC device
manufacturing [125]. Oxides grown in a high pressure ambient have also been found to have significantly reduced
stacking faults, leading to an improved device performance [98].
Multiple studies have shown that silicon is not oxidized at the same rate in each crystalline
direction [122]. Therefore, the crystal orientation of the wafer plays a role in determining the oxide
thickness, as can be seen in Figure 2.11. Oxide growth appears to be faster on (111) oriented silicon
when compared to (100) oriented silicon. In fact, in [122] it is shown that the (111) and (100) orientations
represent the upper and lower bound for oxidation rates, respectively. All other silicon orientations lie between these extrema.
Figure 2.11:
Oxide thickness versus oxidation time for (100) and (111) oriented silicon by wet oxidation at various temperatures.
|
![\includegraphics[width=0.65\linewidth]{chapter_oxidation/figures/oxidation_100_wet_orientation.eps}](img171.png) |
Ligenza [126] argued that the crystal orientation effect on the oxidation rate is due to the differences in the densities
of silicon atoms on the various crystal faces. Since silicon atoms are required in order to generate the oxide, having
a larger number of bondable Si atoms available on the (111) face meant that the oxide would grow faster in the (111) direction, as
is observed experimentally.
L. Filipovic: Topography Simulation of Novel Processing Techniques
![]() O) or
dry (O
O) or
dry (O![]() ) also plays a role in determining the growth rate, in addition to the role played by the
crystal orientation of the silicon wafer.
) also plays a role in determining the growth rate, in addition to the role played by the
crystal orientation of the silicon wafer.
![]() ) environment and the chemical reaction which ensues
is between the solid silicon atoms (Si) on the surface of the wafer and the approaching oxide gas
) environment and the chemical reaction which ensues
is between the solid silicon atoms (Si) on the surface of the wafer and the approaching oxide gas
![\includegraphics[width=0.65\linewidth]{chapter_oxidation/figures/oxidation_100_dry.eps}](img158.png)
![]() O) and the ensuing chemical reaction
is between the water vapor molecules and the solid silicon atoms (Si) on the surface of the wafer, with hydrogen gas (H
O) and the ensuing chemical reaction
is between the water vapor molecules and the solid silicon atoms (Si) on the surface of the wafer, with hydrogen gas (H![]() )
released as a byproduct
)
released as a byproduct
![\includegraphics[width=0.65\linewidth]{chapter_oxidation/figures/oxidation_100_wet.eps}](img160.png)
![\includegraphics[width=0.65\linewidth]{chapter_oxidation/figures/temperature.eps}](img162.png)
![\includegraphics[width=0.65\linewidth]{chapter_oxidation/figures/oxidation_100_wet_orientation.eps}](img171.png)