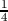 ,
,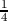 ,
,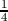 ) with
respect to the original fcc lattice. One unit cell of silicon consists of eight
atoms, where their distribution with reference to Figure 3.1 is described as
below.
) with
respect to the original fcc lattice. One unit cell of silicon consists of eight
atoms, where their distribution with reference to Figure 3.1 is described as
below.
The Miller indices, denoted as h, k, and l, are a symbolic vector representation for the orientation of atomic planes and directions in a crystal lattice. Defining three lattice vectors forming the lattice axes, any crystal plane would intersect the axes at three distinct points. The Miller indices are obtained by taking the reciprocal of the intercepted values. By convention, negative indices are written with a bar over the indices. The adopted nomenclature is as described below [139].
The crystal structure of silicon is classified under the diamond structure [139],
and thus has two atoms in a primitive cell. Germanium, and carbon are
further examples of a diamond structure lattice. On the contrary, the III-V
semiconductors (GaAs, AlAs, InAs, InP etc.) are of zinc blende type. The crystal
lattice of silicon can be represented as two penetrating face centered cubic lattices
(fcc) with the cube side a=0.543nm as portrayed in Figure 3.1. The structure is
visualized as a tetrahedron with four vertices of the first fcc lattice at (0,0,0),
(a/2,0,0), (0,a/2,0) and (0,0,a/2) and an additional atom added to the center
of this tetrahedron. The additional atom is displaced by a(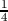 ,
,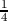 ,
,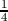 ) with
respect to the original fcc lattice. One unit cell of silicon consists of eight
atoms, where their distribution with reference to Figure 3.1 is described as
below.
) with
respect to the original fcc lattice. One unit cell of silicon consists of eight
atoms, where their distribution with reference to Figure 3.1 is described as
below.