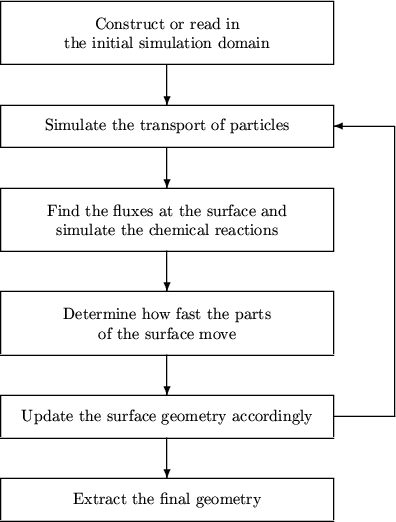
12.2 Overview of Topography Simulation
Figure 12.1:
Overview of the general simulation flow of topography
simulations.
 |
Before immersing into the details of the simulation, a typical CVD
(chemical vapor deposition) process is discussed [111]. The
chemical reaction can happen in the gas phase, where it is called a
homogeneous reaction, or at the boundary between gas and wafer, where
it is called a heterogeneous reaction. Homogeneous reactions are not
desired in CVD processes since they lead to the formation of clusters
and thus to defects in the film. The heterogeneous reactions,
however, yield high quality films and run selectively in areas where
the temperature is high enough. During heterogeneous reactions the
surface of the substrate often acts as a catalyst. Concerning CVD
processes, the following chemical reactions are discerned: pyrolysis
(heterogeneous decomposition reaction), reduction, oxidation, and
hydrolysis.
The whole deposition or etching process, including the reactor scale
processes which were not considered, can be divided into several
parts.
- Transport of the reactants by convection through the reactor to
the deposition region. The reactants are solved in carrier gas.
This step is governed by the Navier-Stokes equation.
- Transport of the reactants from the convective zone through the
boundary layer to the wafer surface.
- Adsorption of the reactants at the wafer surface.
- Surface reaction: dissociation of the molecules, surface
diffusion of the radicals, incorporation of the radicals in the
solid structure, and formation of byproducts.
- Desorption of the byproducts.
- Transport of the byproducts from the wafer surface through the
boundary layer to the convective zone.
- Transport of the byproducts by convection away from the
features.
Here the boundary layer is the small region above the wafer surface
where the velocity of the flow of the gas is between zero at the
surface and the velocity of the flow in the convective zone.
Operating the equipment the temperature, the pressure and the amount
of species entering the reactor can be adjusted. These three
parameters influence the transport near the wafer surface and the
surface deposition reactions which are discussed in two of the
following sections.
The feature scale simulation of deposition and etching processes can
be divided into three main steps. The according simulation flow of a
typical topography simulation is shown in
Figure 12.1.
- The transport of particles in the boundary layer above the wafer
must be simulated. It can happen in the radiosity or diffusion
regime (cf. Section 12.3).
- The fluxes of particles at the wafer surface found in the
previous step determine the chemical reactions that take place at
the wafer surface (cf. Sections 12.5 and
12.6).
- The surface of the wafer changes according to the previous
steps. Describing the wafer surface and its change due to the
fluxes of etchants and particles to be deposited is an important
part of topography simulations and will be discussed in detail in
Chapter 13.
The rest of this chapter and Chapter 13 are
devoted to the simulation of these main steps. Before we go into
details, we recall some chemical facts.
The coupling between gas concentrations 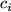 within the reactor space
and the fluxes
within the reactor space
and the fluxes 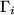 towards the surface is given by the relation
towards the surface is given by the relation
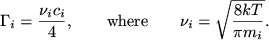 |
(12.1) |
Here 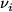 is the average molecular velocity of gas species
is the average molecular velocity of gas species 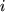 with a molar mass
with a molar mass 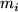 .
.
Relating pressure, volume, and temperature, the state equation of the
ideal gas is
where 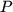 denotes pressure,
denotes pressure, 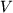 volume,
volume,  the number of moles,
the number of moles,
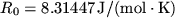 the universal gas
constant and
the universal gas
constant and 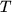 absolute temperature. Using
absolute temperature. Using 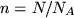 ,
where
,
where 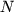 is the number of molecules and
is the number of molecules and 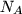 the Avogadro constant,
and introducing the Boltzmann constant
the Avogadro constant,
and introducing the Boltzmann constant 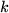 as
as
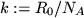 , the state
equation can also be written as
where
, the state
equation can also be written as
where
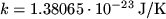 . In the form
. In the form
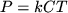 |
(12.2) |
the state equation relates pressure, temperature, and concentration.
As the unit of pressure,
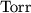 is usually used by equipment
manufacturers. In SI units this is
is usually used by equipment
manufacturers. In SI units this is
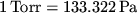 .
.
The classic Langmuir adsorption model [111] describes how gas
adsorption on a solid surface depends on pressure via the equation
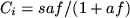 , where
, where 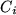 is the concentration of adsorbed gas
molecules,
is the concentration of adsorbed gas
molecules,  a constant,
a constant, 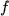 the gas fugacity, and
the gas fugacity, and  the
concentration of potential adsorption sites on the surface.
the
concentration of potential adsorption sites on the surface.
Clemens Heitzinger
2003-05-08

![]() within the reactor space
and the fluxes
within the reactor space
and the fluxes ![]() towards the surface is given by the relation
towards the surface is given by the relation
![]() , where
, where ![]() is the concentration of adsorbed gas
molecules,
is the concentration of adsorbed gas
molecules, ![]() a constant,
a constant, ![]() the gas fugacity, and
the gas fugacity, and ![]() the
concentration of potential adsorption sites on the surface.
the
concentration of potential adsorption sites on the surface.