 |
To describe local oxidation, a model with a smooth transition zone between silicon and silicon dioxide is assumed[16].
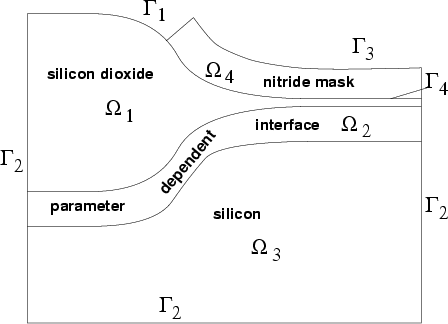
For the definition of the model consider figure [14] as
computation domain ![]() which consists of a pure silicon dioxide
range
which consists of a pure silicon dioxide
range ![]() , an interface range
, an interface range ![]() with a mixture of
silicon and silicon dioxide, a pure silicon range
with a mixture of
silicon and silicon dioxide, a pure silicon range ![]() and a
nitride mask
and a
nitride mask ![]() which is defined on a mesh of its own and is connected
to
which is defined on a mesh of its own and is connected
to ![]() via boundary
via boundary ![]() to transmit mechanical displacements.
For the nitride mask an elastic model is used to calculate its stress-strain
contribution. To describe the different phases of oxygen within the domain
to transmit mechanical displacements.
For the nitride mask an elastic model is used to calculate its stress-strain
contribution. To describe the different phases of oxygen within the domain
![]() a generation/recombination ratio of oxygen
a generation/recombination ratio of oxygen
| (14) |
The generation of silicon dioxide itself is handled by the formulation
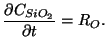 |
(15) |
![$\displaystyle \frac{\partial C_{O}}{\partial t}=div\left[D(\left(\eta(x,t)\right)\cdot grad\left( C_{O}\right) \right] - 2\cdot R_O.$](img100.gif) |
(16) |
For the volumetric expansion we solve the equilibrium condition
| (18) |
As an example two typical three-dimensional effects arising in the corners of the nitride mask have been investigated (Fig. 15). Starting from a pure silicon block the results show, that the introduced model can even handle structures without pad oxide below the nitride mask, which leads to the effect, that the interface meets the nitride layer vertically, which can hardly be solved by algorithms based on a sharp interface formulation.
|