 |
The penetration depth of the implanted particles is mainly determined by the ion energy. The typical energy range used in semiconductor technology is 100 eV for shallow doping used for instance for gate profiling to 200 keV used for the well formation in MOS technology. Higher energies (200 keV - 1.5 MeV) are required for some low dose applications like retrograde wells and very high energy implantations (several MeV) are sometimes used for the generation of triple wells or high dose buried layers. The ion energy is a very well controllable parameter. A modern implanter guarantees a deviation below 1%.
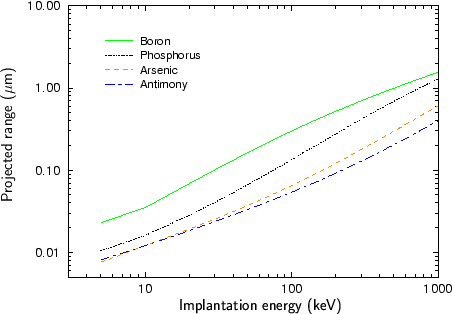
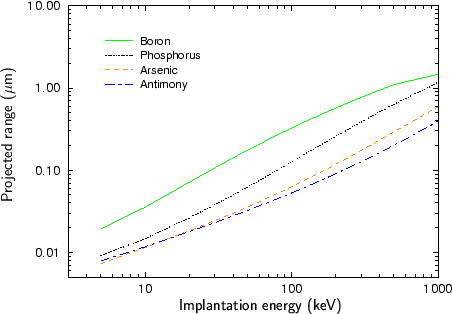
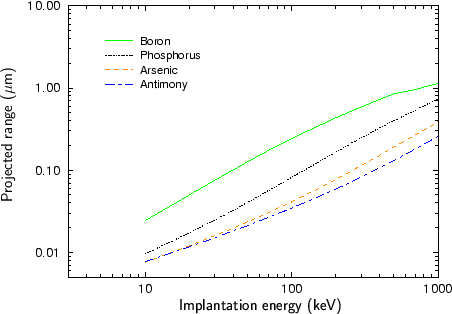
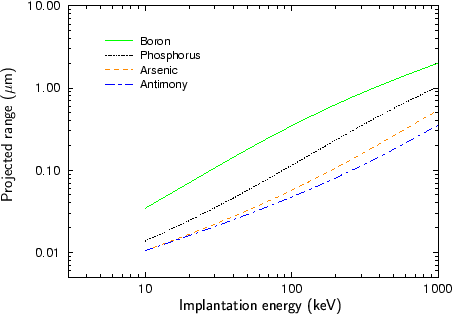
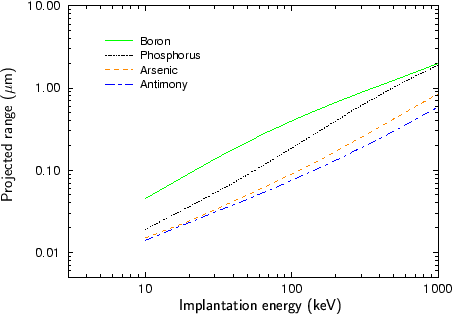
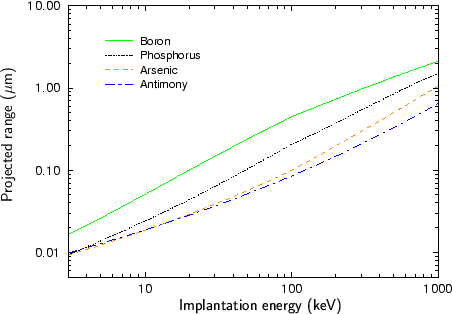
Fig. 2.4 - Fig. 2.9 show experimentally determined projected ranges of some species in various target materials [2], [26], [52], [53], [92]. The projected ranges are extracted from profiles measured by secondary ion mass spectrometry (SIMS). Only amorphous target materials where used for the measurements.
 |
 |
 |
 |
 |
 |
![\begin{figure}\begin{center}
\psfrag{Stopping power}[c][c]{\LARGE\sf Stopping po...
...{\includegraphics{fig/technology/LowStoppingPower.eps}}}\end{center}\end{figure}](img76.gif) |
According to Fig. 2.4 - Fig. 2.9 generally the projected range of an ion is all the higher the lower the mass of the implanted particle. Therefore boron has the largest projected range from the analyzed particle species, while antimony has the shortest projected range.
An exception of this rule can be observed at low energies. Below 20 keV the projected range of antimony is slightly lower than the projected range of arsenic for the target materials silicon, silicon dioxide and the photo-resist AZ-7500. For all other target materials the projected ranges of antimony and arsenic are approximately equal. This effect can be explained by the fact that the stopping of the ions is dominated by nuclear stopping in the lower energy regime while it is dominated by electronic stopping in the high energy regime as will be explained in Sec. 3.3.1. The stopping power due to electronic stopping is proportional to the charge of the ion. Therefore the projected range decreases with increasing ion charge. In principle the stopping power due to nuclear stopping also increases with increasing ion charge, but the nuclear stopping power reaches a maximum at a certain energy and this maximum moves to higher energies if the ion charge is increased. For low ion energies the stopping power can therefore be inversely proportional to the charge of the ion as illustrated in Fig. 2.10.
Another interesting effect is that the difference in the projected ranges becomes smaller also for very high ion energies as can be seen especially in Fig. 2.8 for the ion species boron and phosphorus, and in Fig. 2.9 for the ion species phosphorus and arsenic. The reason for this effect is that also the electronic stopping power reaches a maximum. The position of this maximum is all the lower the lower the mass of the ion.
![]()
![]()
![]()
![]() Previous: 2.2.1 Dopant Species
Up: 2.2 Ion Implantation Process
Next: 2.2.3 Implantation Dose
Previous: 2.2.1 Dopant Species
Up: 2.2 Ion Implantation Process
Next: 2.2.3 Implantation Dose