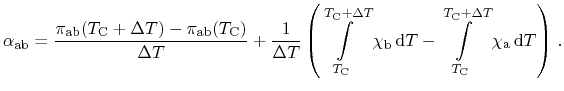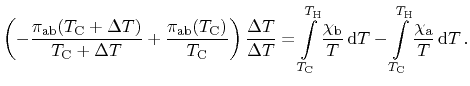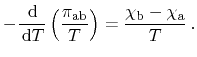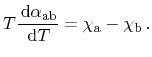As already indicated, the three thermoelectric effects are not independent from
each other and thus the according coefficients are related. In the sequel,
these relations are discussed on the basis of fundamental thermodynamics
[9,10,11].
While all three effects describe reversible phenomena, further two irreversible
processes occur within the structure. First, each electrical current causes
the dissipation of Joule heat when passing a material with a certain
electrical resistance. Second, heat is conducted within the device as
described by Fourier's law.
In the following derivations, the device illustrated in
Fig. 2.1 is considered as electrically short-circuited for the
sake of brevity. Thus, no external electric voltage is induced and no electric
power is dissipated. Furthermore, the cold and the hot contact are connected
to thermal reservoirs. Energy losses by Joule heating are very small and
can be safely neglected. Considering all three thermoelectric effects, the
application of total energy conservation within the entire device including the
reservoirs for a closed loop reads
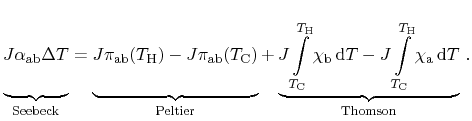 |
(2.10) |
There, the Seebeck effect maintains a driving force causing a current
running throughout the device. This current itself induces the Peltier
effect as well as the Thomson effect. Introducing the temperature
difference
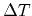 as
as
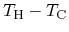 and dividing (2.10) by
and dividing (2.10) by
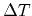 as well as
as well as 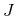 results in
results in
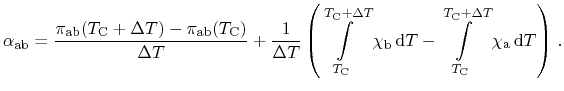 |
(2.11) |
Letting
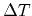 approach zero, the energy relation between the three
effects is obtained
approach zero, the energy relation between the three
effects is obtained
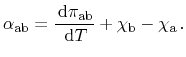 |
(2.12) |
Next, the net change of entropy of the entire structure including the heat
reservoirs can be assumed to be zero due to the neglect of irreversible
processes. Accordingly, contributions from all three effects cancel
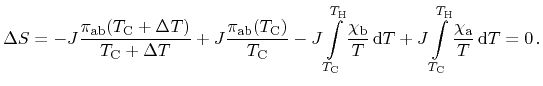 |
(2.13) |
Division of (2.13) by 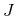 as well as extending the Peltier term
by
as well as extending the Peltier term
by
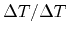 results in
results in
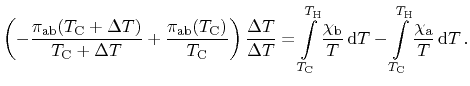 |
(2.14) |
Again letting
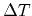 approach zero, the relation between Peltier and
Thomson coefficients is obtained as
approach zero, the relation between Peltier and
Thomson coefficients is obtained as
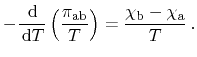 |
(2.15) |
Expansion of the derivative yields a more convenient formulation
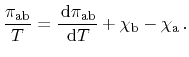 |
(2.16) |
Inserting (2.12) to (2.16) yields the correlation between
Seebeck and Peltier effect, which has already been observed by Thomson
and is well known as the first Kelvin relation
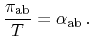 |
(2.17) |
Furthermore, substitution of the Peltier term in (2.16) with
(2.17) yields the correlation between Seebeck and Thomson
coefficients, which is known as the second Kelvin relation
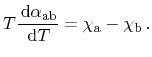 |
(2.18) |
The same result can be obtained from Onsager's reciprocal relations of
irreversible thermodynamics [12] which are a cornerstone within the
description of linear irreversible processes and are applied in
Section 3.5.9 to analyze macroscopic transport models.
M. Wagner: Simulation of Thermoelectric Devices