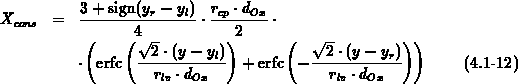4.1 Oxide Growth Model




Next: 4.2 Applications
Up: 4 Oxidation
Previous: 4 Oxidation
Physical models for the oxidation process are based on the works of Deal and
Grove [Dea65], Chin et al. [Chi83], Kao et al. [Kao88] and
Rafferty [Raf91].
The diffusion of the oxidant is governed by (4.1-1), where
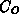 is the oxidant concentration and
is the oxidant concentration and 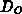 is the oxidant's diffusion
coefficient in
is the oxidant's diffusion
coefficient in 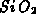 . The boundary conditions follow the Deal-Grove
model (4.1-2), with
. The boundary conditions follow the Deal-Grove
model (4.1-2), with 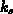 the interface reaction coefficient,
the interface reaction coefficient,
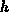 the mass transfer coefficient and
the mass transfer coefficient and 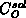 the oxidant solubility in
the oxidant solubility in
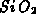 .
.
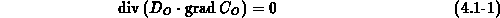
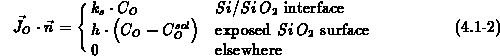
For process temperatures above 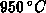 viscous flow of oxide is commonly
assumed [Kao88], [Umi89]. The slow incompressible (
viscous flow of oxide is commonly
assumed [Kao88], [Umi89]. The slow incompressible (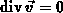 ) flow of oxide is expressed by a creeping flow
equation (4.1-3) in which the viscous force is balanced with
the pressure gradient. There
) flow of oxide is expressed by a creeping flow
equation (4.1-3) in which the viscous force is balanced with
the pressure gradient. There 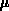 denotes the oxide viscosity,
denotes the oxide viscosity, 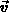 is
the oxide velocity and
is
the oxide velocity and 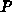 is the hydrostatic pressure.
is the hydrostatic pressure.
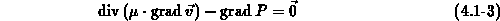
The following boundary conditions apply to (4.1-3). At the
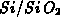 interface the velocity is determined from the net volume
expansion
interface the velocity is determined from the net volume
expansion 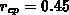 , i.e. the ratio of consumed
, i.e. the ratio of consumed 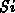 to produced
to produced
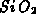 , and
, and 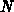 is the number of oxidant molecules in a unit volume of
oxide (4.1-4). At the free oxide surface and at an eventual
nitride/oxide interface pressure equilibrium is assumed.
is the number of oxidant molecules in a unit volume of
oxide (4.1-4). At the free oxide surface and at an eventual
nitride/oxide interface pressure equilibrium is assumed.
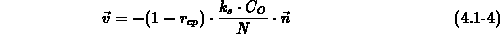
Stress effects on the oxide growth are usually considered by
stress-dependent surface reaction 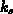 , oxidant diffusivity
, oxidant diffusivity 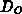 , and
viscosity
, and
viscosity 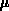 , where
, where 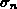 ,
, 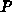 , and
, and  denote the normal stress,
hydrostatic pressure and shear stress, respectively. The volumes
denote the normal stress,
hydrostatic pressure and shear stress, respectively. The volumes 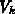 ,
, 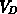 and
and 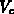 , despite their physical meaning, and
, despite their physical meaning, and 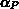 are used as
fitting coefficients.
are used as
fitting coefficients.
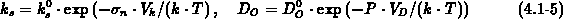
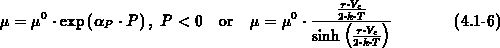
In this model, in the one-dimensional case the motion of the oxide is
stress-free, uniform and perpendicular to the surface. According to
equations (4.1-1), (4.1-2)
and (4.1-4) Deal and Grove ended up with a relation for the oxide
thickness 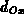 (4.1-7).
(4.1-7).
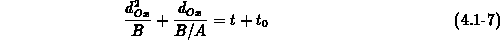
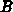 is referred to as parabolic growth rate, and
is referred to as parabolic growth rate, and 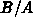 is the linear growth
rate. For these two parameters there exist well established models for
dry and wet oxidation including pressure dependence, chlorine dependence and
dry oxide kinetics [Ho83a]. From
is the linear growth
rate. For these two parameters there exist well established models for
dry and wet oxidation including pressure dependence, chlorine dependence and
dry oxide kinetics [Ho83a]. From 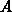 and
and 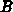 we derive the stress-free
parameters
we derive the stress-free
parameters 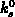 and
and 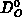 , assuming the mass transfer in the gas phase
being effective, i.e.
, assuming the mass transfer in the gas phase
being effective, i.e. 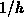 is negligible compared to
is negligible compared to 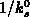 .
.
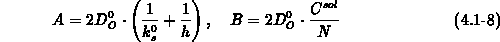
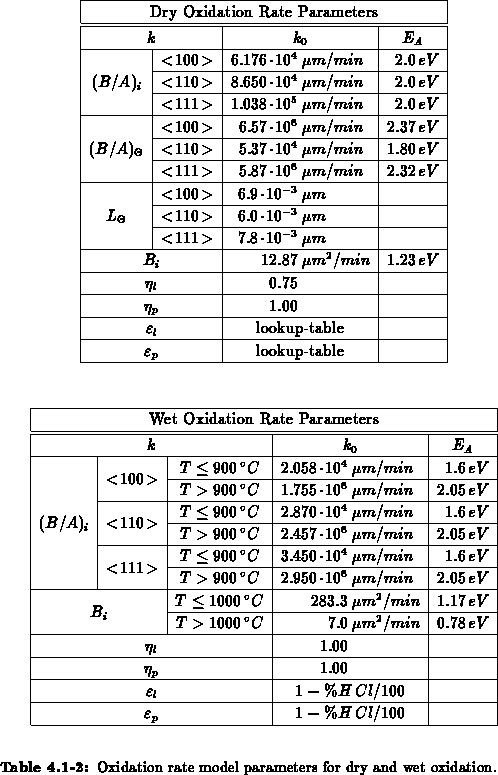
We have implemented the models (4.1-9) and (4.1-10) for
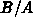 and
and 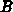 , respectively.
, respectively. 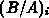 and
and 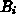 are the intrinsic
orientation dependent linear and parabolic rate constants, by
are the intrinsic
orientation dependent linear and parabolic rate constants, by 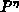 we
take the oxidant pressure dependence into account,
we
take the oxidant pressure dependence into account,  describes
the chlorine dependence, and
describes
the chlorine dependence, and 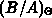 accounts for the anomalous fast
growth of thin oxides (
accounts for the anomalous fast
growth of thin oxides (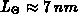 ).
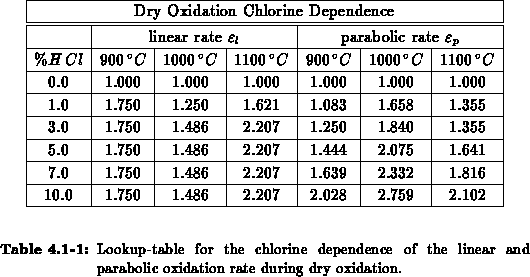
Tables 4.1-1 to 4.1-2 summarize the implemented
parameter values. Most of the parameter values are modeled by Arrhenius laws
(
).
Tables 4.1-1 to 4.1-2 summarize the implemented
parameter values. Most of the parameter values are modeled by Arrhenius laws
(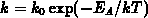 ).
).
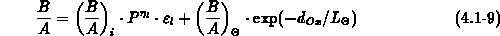
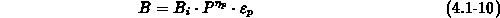
These parameter models for 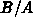 and
and 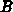 are used on the one hand to calibrate
two-dimensional oxidant flow simulations, and on the other hand they are
the basis for one- and two-dimensional analytical oxide shape calculations.
The one-dimensional oxide growth increment
are used on the one hand to calibrate
two-dimensional oxidant flow simulations, and on the other hand they are
the basis for one- and two-dimensional analytical oxide shape calculations.
The one-dimensional oxide growth increment 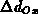 is calculated
from the actual oxide thickness
is calculated
from the actual oxide thickness 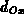 , the time increment
, the time increment 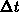 and
the actual parameters
and
the actual parameters 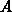 and
and 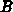 using (4.1-11), a numerically
uncritical solution of (4.1-7).
using (4.1-11), a numerically
uncritical solution of (4.1-7).
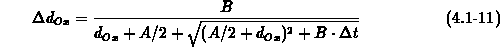
As two-dimensional analytical model we have implemented (4.1-12)
for the amount of consumed silicon 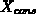 to cover the four basic LOCOS
cases shown in Figure 4.1-1. The lateral positions
to cover the four basic LOCOS
cases shown in Figure 4.1-1. The lateral positions 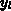 and
and 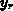 are a left and right mask edge, respectively, and the fit
parameter
are a left and right mask edge, respectively, and the fit
parameter 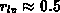 describes the ratio of lateral to
vertical oxide growth.
describes the ratio of lateral to
vertical oxide growth.
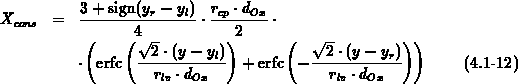




Next: 4.2 Applications
Up: 4 Oxidation
Previous: 4 Oxidation
Martin Stiftinger
Wed Oct 19 13:03:34 MET 1994
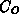 is the oxidant concentration and
is the oxidant concentration and 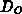 is the oxidant's diffusion
coefficient in
is the oxidant's diffusion
coefficient in 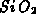 . The boundary conditions follow the Deal-Grove
model (4.1-2), with
. The boundary conditions follow the Deal-Grove
model (4.1-2), with 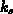 the interface reaction coefficient,
the interface reaction coefficient,
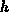 the mass transfer coefficient and
the mass transfer coefficient and 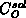 the oxidant solubility in
the oxidant solubility in
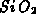 .
.




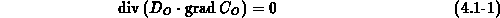
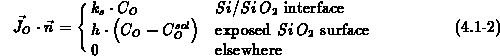
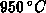 viscous flow of oxide is commonly
assumed
viscous flow of oxide is commonly
assumed 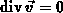 ) flow of oxide is expressed by a creeping flow
equation (
) flow of oxide is expressed by a creeping flow
equation (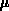 denotes the oxide viscosity,
denotes the oxide viscosity, 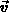 is
the oxide velocity and
is
the oxide velocity and 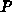 is the hydrostatic pressure.
is the hydrostatic pressure.
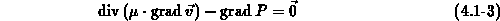
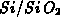 interface the velocity is determined from the net volume
expansion
interface the velocity is determined from the net volume
expansion 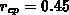 , i.e. the ratio of consumed
, i.e. the ratio of consumed 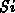 to produced
to produced
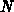 is the number of oxidant molecules in a unit volume of
oxide (
is the number of oxidant molecules in a unit volume of
oxide (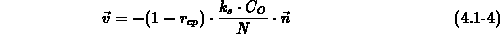
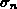 ,
,  denote the normal stress,
hydrostatic pressure and shear stress, respectively. The volumes
denote the normal stress,
hydrostatic pressure and shear stress, respectively. The volumes 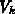 ,
, 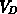 and
and 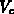 , despite their physical meaning, and
, despite their physical meaning, and 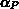 are used as
fitting coefficients.
are used as
fitting coefficients.
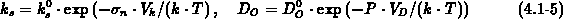
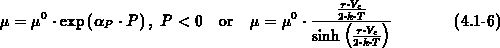
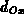 (
(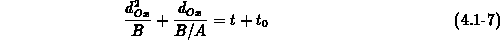
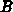 is referred to as parabolic growth rate, and
is referred to as parabolic growth rate, and 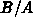 is the linear growth
rate. For these two parameters there exist well established models for
dry and wet oxidation including pressure dependence, chlorine dependence and
dry oxide kinetics
is the linear growth
rate. For these two parameters there exist well established models for
dry and wet oxidation including pressure dependence, chlorine dependence and
dry oxide kinetics 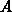 and
and 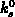 and
and 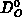 , assuming the mass transfer in the gas phase
being effective, i.e.
, assuming the mass transfer in the gas phase
being effective, i.e. 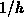 is negligible compared to
is negligible compared to 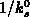 .
.
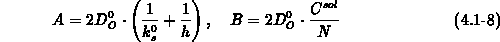
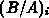 and
and 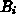 are the intrinsic
orientation dependent linear and parabolic rate constants, by
are the intrinsic
orientation dependent linear and parabolic rate constants, by 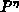 we
take the oxidant pressure dependence into account,
we
take the oxidant pressure dependence into account,  describes
the chlorine dependence, and
describes
the chlorine dependence, and 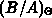 accounts for the anomalous fast
growth of thin oxides (
accounts for the anomalous fast
growth of thin oxides (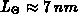 ).
Tables
).
Tables 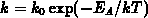 ).
).
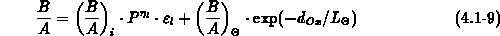
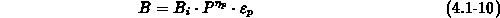
 is calculated
from the actual oxide thickness
is calculated
from the actual oxide thickness 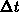 and
the actual parameters
and
the actual parameters 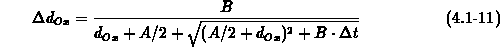
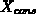 to cover the four basic LOCOS
cases shown in Figure
to cover the four basic LOCOS
cases shown in Figure 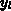 and
and 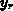 are a left and right mask edge, respectively, and the fit
parameter
are a left and right mask edge, respectively, and the fit
parameter 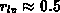 describes the ratio of lateral to
vertical oxide growth.
describes the ratio of lateral to
vertical oxide growth.