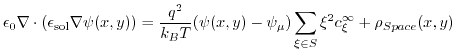 |
(6.6) |
From (6.6) two important properties can be gained. Firstly, the Debye length
![]() :
:
![\includegraphics[width=0.7\textwidth]{figures/debyelength.ps}](img850.png)
|
The Debye length
![]() is also an important parameter in the sense of giving a rule of thumb on how far a charged macromolecule can be away from the dielectric-electrolyte interface without being completely shielded by the ions in the electrolyte. It is also a measure of how much of the macromolecule's charge is actually able to couple to the semiconductor without being screened for very large macromolecules (e.g. DNA).
The Debye length
is also an important parameter in the sense of giving a rule of thumb on how far a charged macromolecule can be away from the dielectric-electrolyte interface without being completely shielded by the ions in the electrolyte. It is also a measure of how much of the macromolecule's charge is actually able to couple to the semiconductor without being screened for very large macromolecules (e.g. DNA).
The Debye length
![]() affects the double layer thickness. Increasing the concentration or valence of the counterions compresses the double layer and raises the potential gradient.
affects the double layer thickness. Increasing the concentration or valence of the counterions compresses the double layer and raises the potential gradient.
The second parameter is already introduced in (6.8) and describes the ionic strength of the electrolyte defined as:
The ionic strength of a solution is a measure of the concentration of ions in the solution. The ionic compounds (e.g salts), dissociate into ions, when they are dissolved in water. Ionic strength is one of the main characteristics of a solution with dissolved ions, which influences many important properties like, the dissociation or solubility of different salts and the double layer thickness. The ionic strength is utilized to describe the strong deviations from the ideality typically experienced in ionic solutions via the Debye-Hückel theory.
It is furthermore related to electrokinetic phenomena, electroacoustic phenomena in colloids and other heterogeneous systems and linked to the electric double layer (eq. (6.8)). The Debye length
![]() is inversely proportional to the square root of the ionic strength Fig. 6.3.
is inversely proportional to the square root of the ionic strength Fig. 6.3.
In order to minimize changes during a titration6.2 in the activity quotient of solutes at lower concentrations, media with high ionic strength are employed. Natural waters such as seawater have a non-zero ionic strength due to the presence of dissolved salts which significantly affects their properties.