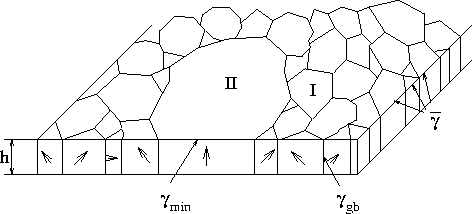
Figure 3.2-3: Polysilicon film with thickness h composed of columnar primary grains I and an average surface energy
Generally, grain growth can be characterized by two mechanisms termed primary recrystallization and secondary recrystallization. Recrystallization can only occur in materials with a high number of lattice defects or natural disorder. The process of primary recrystallization is driven by the defect energy, whereas the secondary recrystallization is directly deduced from the grain boundary energy. In the case of primary recrystallization the transition of the grains is continuous, which means that the grains are growing from a large amount of tiny grains to a small amount of large grains, without any interstates. Polysilicon, however, is primarily growing via secondary grain growth. Some existing primary grains (see I in Fig. 3.2-3) have preferred energetic properties and start to grow with an extraordinary growth rate. During the growth process they coalesce with adjacent smaller grains to form secondary grains (see II in Fig. 3.2-3).

Figure 3.2-3: Polysilicon film with thickness
h composed of columnar primary grains I and an average surface
energy ![]() and a single secondary grain II with a
minimum surface energy
and a single secondary grain II with a
minimum surface energy ![]() .
.
When secondary grains are formed the surface energy is minimized. Now we
want to calculate the migration of the grain boundaries from thermodynamic
concepts of surface energy anisotropy and secondary grain growth
[Tho85] [Kal90]. To model the growth of secondary grains, we
assume a cylindrical grain with predefined radius ![]() and thickness h
growing into a matrix of normal grains. The change in Helmholtz free energy
and thickness h
growing into a matrix of normal grains. The change in Helmholtz free energy
![]() for this transformation is given by (3.2-2), where
for this transformation is given by (3.2-2), where ![]() is the surface energy anisotropy,
is the surface energy anisotropy, ![]() the number of normal grains
per unit volume,
the number of normal grains
per unit volume, ![]() the grain boundary area and
the grain boundary area and ![]() the
average grain boundary energy.
the
average grain boundary energy.
Note, that the first two terms in (3.2-2) represent energies driving normal grain growth, while the third term represents a barrier for grain growth, due to the grain boundary energy. Surface energy and grain boundary energy can act as driving force of grain growth on the one hand or as energetic barrier on the other hand. All grains try to minimize their single surface energy, hence, this will lead to films composed of large secondary grains with uniform crystallographic texture.
From basic rate theory, the rate of atomic transfer of dopants from lattice
sites of one grain to those of a neighbor grain is given by a complementary
Arrhenius law in (3.2-3),where ![]() denotes a jump frequency for atoms
at the boundary, and
denotes a jump frequency for atoms
at the boundary, and ![]() is the difference of the electrochemical
potential on either side of the boundary which can be seen as an energetic
limit for the dopants to diffuse into the neighboring grain.
is the difference of the electrochemical
potential on either side of the boundary which can be seen as an energetic
limit for the dopants to diffuse into the neighboring grain.
Under constant pressure and volume the electrochemical potential is given by (3.2-4). The boundary migration G, which can be seen as a growth rate, is obtained from (3.2-5).
N denotes Avogadro's number, ![]() the atomic volume,
the atomic volume, ![]() the change in Helmholtz free energy given by (3.2-2), and
the change in Helmholtz free energy given by (3.2-2), and ![]() the thickness of the boundary. The jump frequency
the thickness of the boundary. The jump frequency ![]() can be expressed in
terms of the temperature dependent diffusivity. The growth rate is given by
(3.2-6), where
can be expressed in
terms of the temperature dependent diffusivity. The growth rate is given by
(3.2-6), where ![]() is the concentration dependent diffusivity of
all mobile dopants.
is the concentration dependent diffusivity of
all mobile dopants.
The grain growth rate depends on the local doping conditions via the dopant diffusivity. Thus, the average grain size becomes non-uniform within the doped polysilicon film, which agrees with morphological investigations [Wad87]. It was also found, that polysilicon shows anisotropic diffusion properties. Due to the high diffusivity in the grain boundaries, the main diffusion path is given by the grain boundaries. The spatial arrangement of the grains and their shape determines the degree of anisotropy.
The grain growth process depends also on the local stress induced by the
non-planar topology of the polysilicon film. In regions with high stress
rates, like in film corners, grain growth is reduced [Jon91]
[Mei82]. The stress information can be obtained from a previous
deposition simulation. To model such phenomena, (3.2-6) has to be
extended by the stress term S, as given in (3.2-7),
where ![]() is a scaling factor.
is a scaling factor.
Details of the induced stress and the initial setup obtained from the deposition process are presented in Section 4.3.5.